Today Is Rare Disease Day
There are over 300 million people in the world living with a rare condition. Over 6000 rare diseases are characterized by a diverse amalgamation of disorders and symptoms, varying from patient to patient. There are often no existing cures to these ailments, contributing to the level of pain and suffering ensured by carers, family members, and the patients themselves. These factors mean that individuals are more likely to face misdiagnosis, treatment inequality and isolation due to the nature of their disease.
Misdiagnosis and delayed treatment also contributes to a decreased quality of life and advanced progression of the disease, as rare conditions often manifest in relatively common symptoms,
Rare Disease Day was created with the ultimate goal of raising awareness and achieving equitable access to diagnosis, treatment, health, social care and social opportunity for patient populations. Since 2019, Rare Disease Day has contributed to awareness events in over 100 countries worldwide, building a support network at local, national, and international levels.

Diagnosis of Gastrinoma
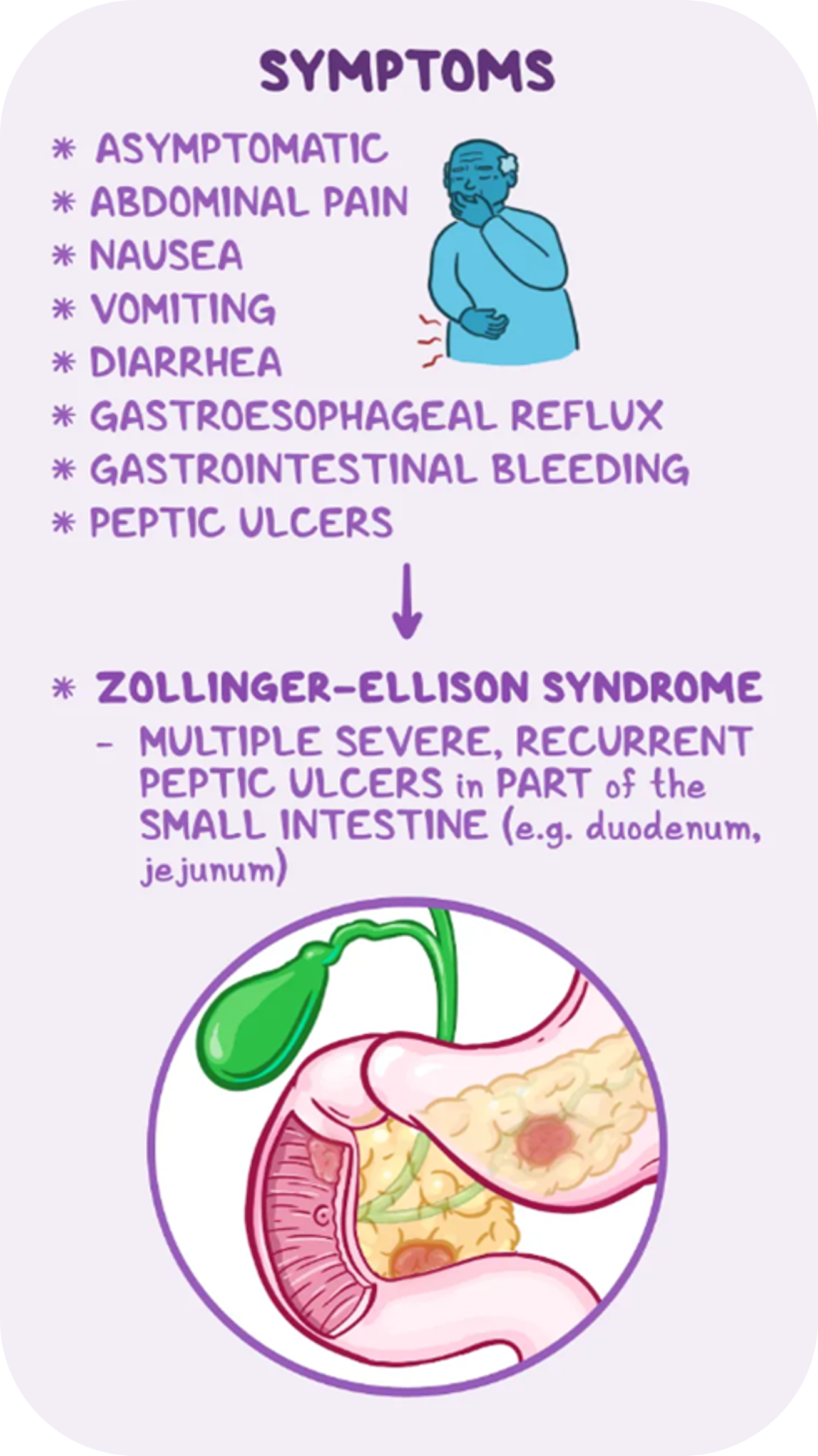
Gastrinoma is a rare neuroendocrine tumor (NET) that can originate from G cells in the pancreas, stomach or small intestine. G cells produce the hormone gastrin, which in turn stimulates the secretion of gastric acid in the stomach. Since gastrinomas are made up of G cells, these tumors continuously overproduce and release the hormone gastrin, which in turn stimulate the parietal cells in the stomach to secrete large amounts of gastric acid. Ultimately, the excess gastric acid can lead to erosion or ulcers in the mucosa of the stomach or small intestine.
Protocol
To perform a Zollinger-Ellison Syndrome Test follow this procedure:
- The patient should have fasted for at least 12 hours prior to beginning the test.
- Prior to injection of ChiRhoStim® (Human Secretin for injection), two blood samples are drawn for determination of fasting serum gastrin levels (baseline values).
- Administer ChiRhoStim® (Human Secretin for injection) at a dose of 0.4 mcg/kg of body weight is injected intravenously over 1 minute;
- Post-injection blood samples are collected after 1, 2, 5, 10, and 30 minutes for determination of serum gastrin concentrations.
- Gastrinoma is strongly indicated in patients who show an increase in serum gastrin concentration of at least 110 pg/mL over basal level on any of the post secretin injection samples.
Citations
Johnson, L. D. (2014). Gastrointestinal Physiology (8 edition). Philadelphia, PA: Elsevier.
Riddell, R. & Jain, D. (2014). Lewin, Weinstein, and Riddell’s Gastrointestinal Pathology and Its Clinical Implications (2 edition). Philadelphia, PA: Lippincott.
Singh, G., Mulji, N. J., & Jialal, I. (2020). Multiple Endocrine Neoplasia Type I (MEN I, Werner Syndrome). In StatPearls. Retrieved August 3, 2020, from https://www.ncbi.nlm.nih.gov/books/NBK536980/
Whittlesea, C. & Hodson, K. (2019). Clinical Pharmacy and Therapeutics (6 edition). Oxford, UK: Elsevier.
Jensen RT, Ito T. Gastrinoma. 2020 Nov 21. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Hofland J, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000–. PMID: 25905301.
